Clinicaltrials.gov is a US-based website that requires companies and investigators to post information about clinical trials in a public forum. All US trials should be posted within about 3 weeks of when the sponsor (company) or investigator plans to start dosing (giving the test medication) the first patient. The site is organized by a simple listing, by topic, by geographic map, or specific search details or search terms. You can search by various topics, such as disease type (or condition which is the term that clinicaltrials.gov uses), previously treated or untreated (treatment naïve), drug or drugs being studied (also known as interventions on clinicaltrials.gov), phases of clinical trials, like phase I, II, and III. (See https://cllsociety.org/2016/03/trial-phases/ for an explanation from the NIH about the different trial phases). It will also help you search for trials that are open and seeking or recruiting new patients compared to those that are running but not looking for new patients or those that are already completed.
To help get you familiar with some terms commonly used on the website, here is a glossary to guide you:
Definitions:
Prospective: patients will be treated in the study and the information that is generated will be new. This is not analyzing old data or also known as retrospective.
Multi-center: the study is conducted at more than one hospital, as opposed to single center
Placebo controlled: one group of patients gets the active study drug alone or in combination with other drugs that are known to work for the disease under study and the other group gets a sugar pill or placebo along with the other drugs known to work in the disease. In this way the true treatment effect of the study drug can be determined and compared to the results of the usual drugs used to treat the disease to determine if the new drug has improvement or side effects over the traditional treatment.
Study arm: Studies can have one or more arms with different treatment approaches in each arm.
Experimental arm: This portion of the study group includes patients who will get the drug being studied either alone or in combination with the usual treatment for the disease
Control arm: This portion of the study group includes patients who do NOT get the study drug but get either medication usually used to treat the condition or a “sugar pill” or placebo if there is no treatment for this condition. In cancer trials, patients are never in an arm where they won’t get an approved appropriate therapy if there is an accepted therapeutic option that has a chance to help. The “control” treatment should be carefully considered before entering any trial where you might end up in that arm.
Double Blind: In this type of trial both the doctor and patient do not know whether the patient is getting study drug or placebo during the study.
Study type: An interventional study tests a new drug or procedure unlike an observational study where patients get their normal treatment and are observed for results.
Randomized: Each patient is assigned a drug treatment, such as A or B, by a computer generated program to insure there is no bias by the doctor as to which patient gets which treatment. This is done to help make sure the study results can be applied to all patients like those in the study
Primary Outcomes Measure: This is the primary objective or question the study is trying to answer. An example might be how many respond or drug x vs drug y after treatment with the study drug.
Secondary Outcomes Measure: These are other questions that the study tries to answer but are of lesser importance than the primary outcome measure.
How to Search
To start your search, go the website: www.clinicaltrials.gov. As of June 7, there are a total of 246,603 studies listed in this website with 42,898 recruiting patients with 56% outside the US (200 countries), 38% inside the US and 5% global. There is a section for patients and families on the bottom left hand side of the website to search for trials.
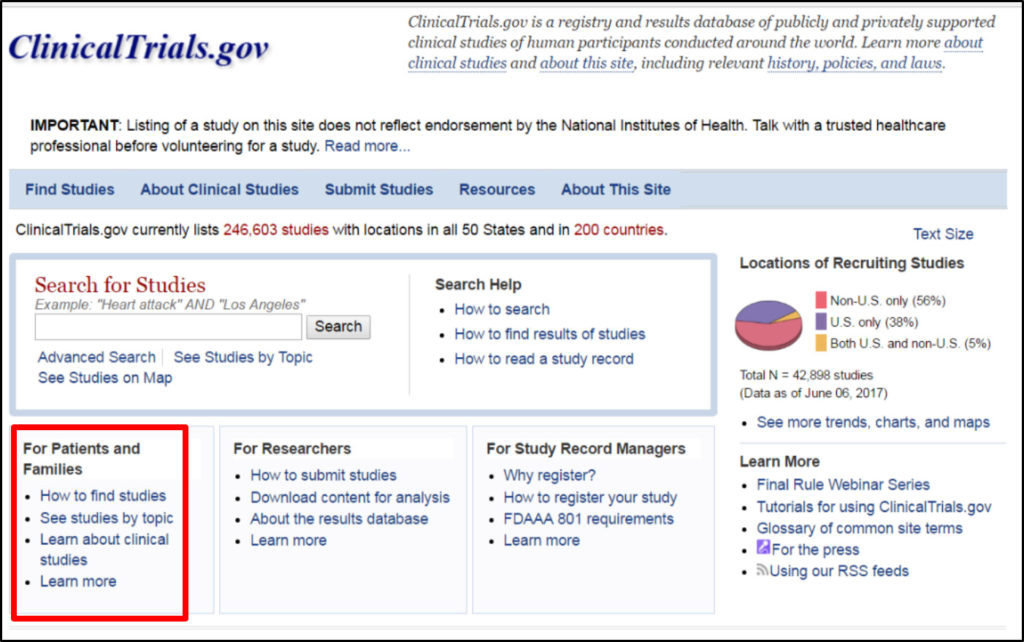
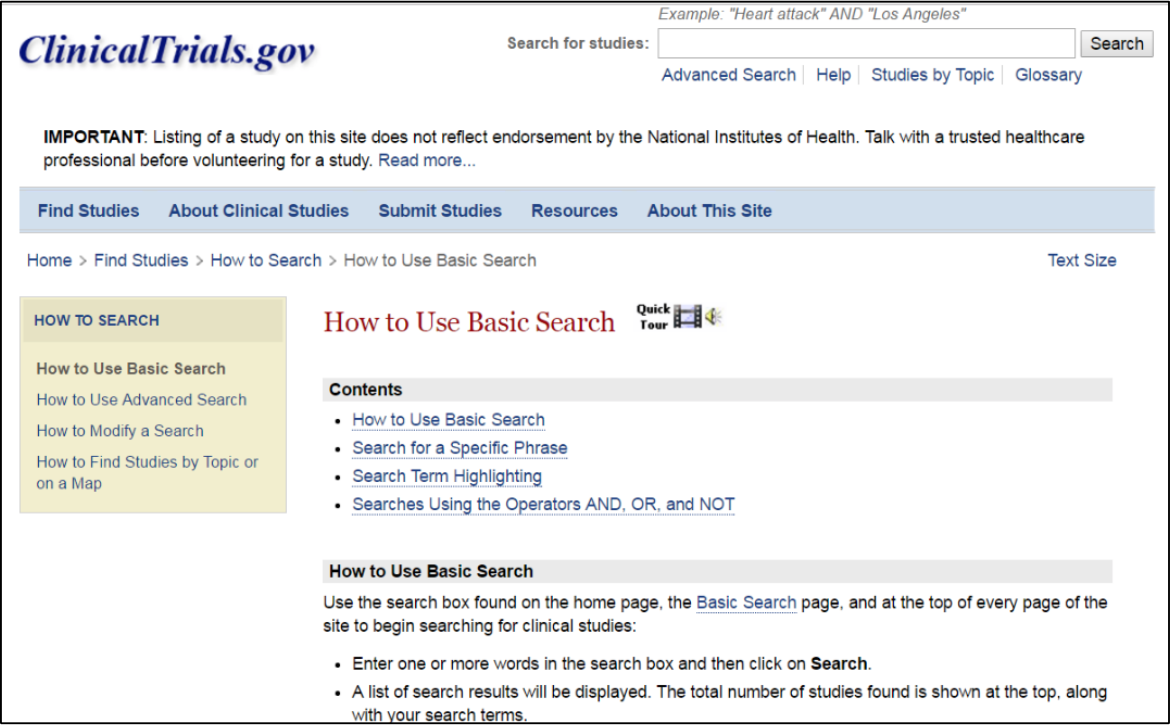
By clicking this link you will be taken to a page on how to use a basic search, searching for a specific phrase, search term highlighting and doing a search with the words, AND, OR and NOT (use all caps when using these conjunction terms). If you want to do a search using more than one word, use AND, OR, or NOT. Use AND when you want all the results for your search term. For example, you can do a search using the linkers such as CLL AND Fludarabine. Use OR, when you want results for any of the words connect by OR, for example idelalisib or venetoclax. Use NOT when you want to exclude a term from your search.
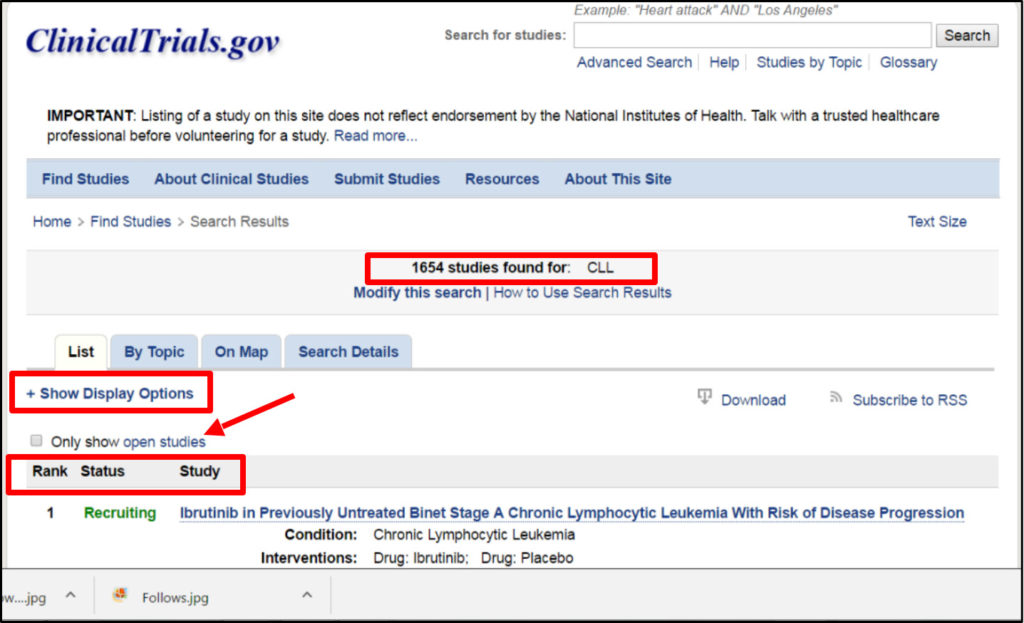
Once you enter a search term, you will be shown a list of results, which shows the total number of studies found at the time. The first column lists the order of the studies that most closely match the term you searched. The status column indicates whether the study is open and looking for new patients or closed to new patients. If you only want a search with specific terms, put parentheses around the words like “newly diagnosed CLL”. Sometimes medical terminology is quite long and complicated so if you use parentheses, they can help you find search results that contain exactly those words like “complex karyotype”. If you use the search term highlighting function, it shows keywords that are an exact match to your search terms in pink and synonyms or words that mean something very similar in yellow.
Just below where it says: + Show display Options, most often you would want to click on the box that says Only show open studies to limit what is displayed to trials that are still active. However this does not always mean that they are presently recruiting new participants.
Now that you know how to search, how do you read the results? Once you have a list of studies for the search you are conducting, then you can click on the name of the study. This page will have an indication after the name of the study if the trial is still recruiting patients or not. It will also have information of the clinical trial identification number, when the trial was first listed or received, when it was lasted updated (and verified). There are three ways to view the trial, in full text, in a tabular or table view and if study results are posted, by study results. Some find the tabular view easiest to view but the default view is the full text.
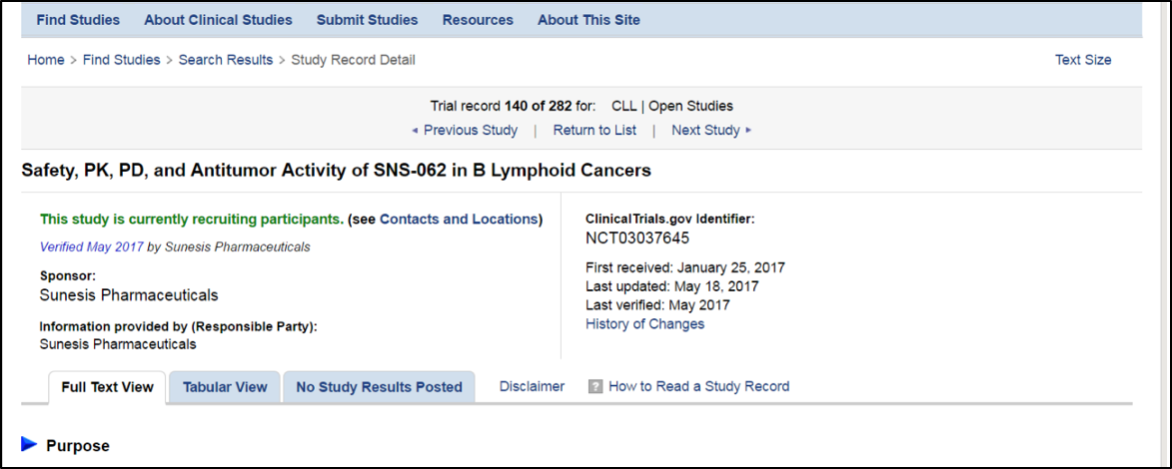
The first set of information is tracking information which you may or may not want to review as the same information is included in the descriptive information lower down. If you are adventurous and want to look at the tracking information, you will come to a section on the primary and secondary outcome measures that state the questions the study is trying to answer. The next information you will see is the total number of patients that are planned to be enrolled in the study, when the study is starting and when the study is expected to be completed. If the study has changed, there may be a listing of the history of study changes.
The next section is called Descriptive Information which is probably the easiest section to read. It includes the brief and full titles, summary of the study and then a detailed description of what questions the study is designed to answer. It will tell you what type of study the trial is and what phase. Phase 1 is the earliest stage, usually designed to learn about how safe the drug is in a small number of patients and to find the right dose for patients with the disease being studied. Phase 2 is get preliminary information on how well the drug works for the disease in a larger number of patients and phase 3 is usually an even bigger set of patients comparing the new treatment to another treatment that is approved to treat the disease. If there is any information published on the study, this may be provided in the publications section.

In the next part of the page is there is a section on Recruitment Information. This section tells you if the study is open and enrolling new patients, when the study opens or is scheduled to be open and when it is expected to be completed.
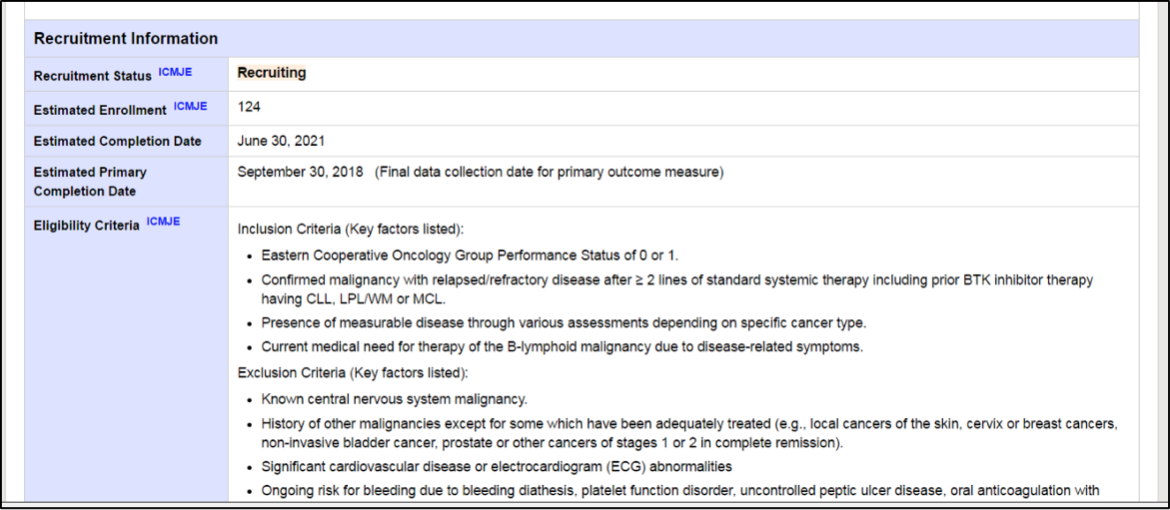
Eligibility criteria are used to create a group of patients with similar disease and patient characteristics to understand how a drug works in a specific population. Inclusion criteria are those requirements that patients have to meet to in order to be included in a trial, such gender, an age range, disease type, etc. Patients are not allowed into a trial if they have exclusion criteria, or certain aspects about either certain demographic identifiers, like age or gender, disease characteristics, such as having a form of disease that cannot be measured by traditional accepted guidelines, have certain risk factors, other medical problems, or types or number of prior treatments.
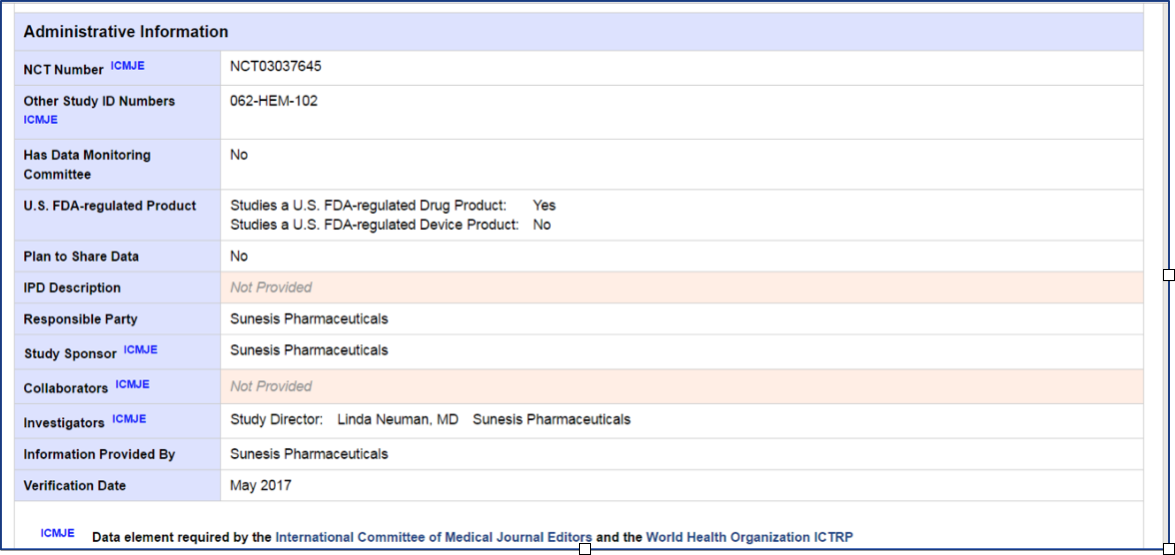
The last part of the page has Administrative Information about what doctor is leading the study, a list of all the doctors who are that are participating in the study and where the study is being conducted. To find the a group of the sites conducting the trial and contact information for the trial, it is best to go back to the text tab and scroll down to the Contacts and Locations section. Here you will find the study centers that are participating and enrolling patients and a phone number to call if you are interested in the trial.
Originally published in The CLL Tribune Q2 2017.

















